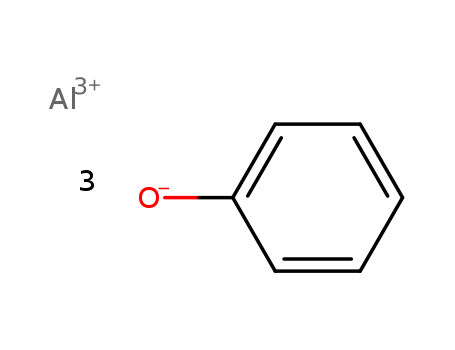
- Chemical Name:ALUMINUM PHENOXIDE
- CAS No.:15086-27-8
- Molecular Formula:C6H6 O . 1/3 Al
- Molecular Weight:306.297
- Hs Code.:2907199090
- Mol file:15086-27-8.mol
Synonyms:Aluminumphenoxide (6CI,7CI); Phenol, aluminum salt (8CI,9CI); Aluminum phenate;Aluminum phenolate; Aluminum triphenolate; Aluminum triphenoxide;Triphenoxyaluminum