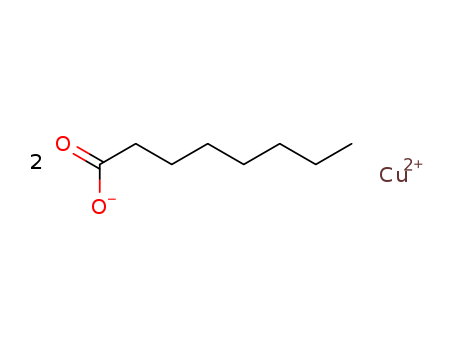
- Chemical Name:COPPER CAPRYLATE
- CAS No.:3890-89-9
- Molecular Formula:2C8H15O2*Cu
- Molecular Weight:349.958
- Hs Code.:
- Mol file:3890-89-9.mol
Synonyms:Octanoicacid, copper(2+) salt (8CI,9CI); Copper caprylate; Copper dioctanoate; Copperdioctoate; Copper octanoate; Copper(II) octanoate; Copper(II) octoate; Cupricoctanoate