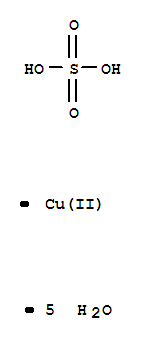
- Chemical Name:Cupric Sulfate
- CAS No.:7758-99-8
- Deprecated CAS:139939-69-8,131540-94-8
- Molecular Formula:CuSO4.5(H2O)
- Molecular Weight:159.61
- Hs Code.:28332500
- European Community (EC) Number:231-847-6,616-477-9
- ICSC Number:0751
- UN Number:3288,3077
- UNII:KUW2Q3U1VV
- DSSTox Substance ID:DTXSID6034479
- Nikkaji Number:J3.756G
- Wikipedia:Copper(II) sulfate,Copper(II)_sulfate
- Wikidata:Q107184
- NCI Thesaurus Code:C65354,C83640
- RXCUI:21579,1999541
- Mol file:7758-99-8.mol
Synonyms:Blue Vitriol;Copper Sulfate;Cupric Sulfate;Sulfate, Copper;Sulfate, Cupric;Vitriol, Blue


 Xn,
Xn,  N,
N,  Xi
Xi


