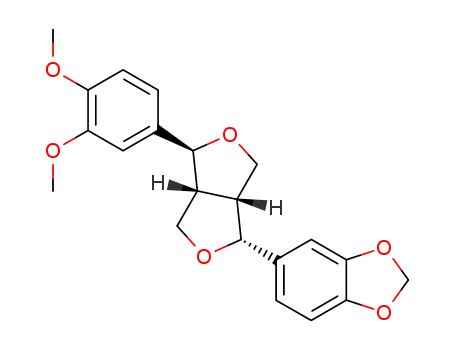
Chemical Property of Kobusin
Edit
Chemical Property:
- Vapor Pressure:7.05E-10mmHg at 25°C
- Boiling Point:506.4°Cat760mmHg
- Flash Point:208.9°C
- PSA:55.38000
- Density:1.265g/cm3
- LogP:3.50770
- XLogP3:2.8
- Hydrogen Bond Donor Count:0
- Hydrogen Bond Acceptor Count:6
- Rotatable Bond Count:4
- Exact Mass:370.14163842
- Heavy Atom Count:27
- Complexity:515
- Purity/Quality:
-
≥98% *data from raw suppliers
(+)-Kobusin *data from reagent suppliers
Safty Information:
- Pictogram(s):
- Hazard Codes:
- MSDS Files:
-
SDS file from LookChem
Useful:
- Canonical SMILES:COC1=C(C=C(C=C1)C2C3COC(C3CO2)C4=CC5=C(C=C4)OCO5)OC
- Isomeric SMILES:COC1=C(C=C(C=C1)[C@@H]2[C@H]3CO[C@@H]([C@H]3CO2)C4=CC5=C(C=C4)OCO5)OC
-
Uses
(+)-Kobusin is a lignan isolated from the bark of Magnolia kobus and inhibits lipopolysaccharide (LPS)-stimulated nitric oxide (NO) production.