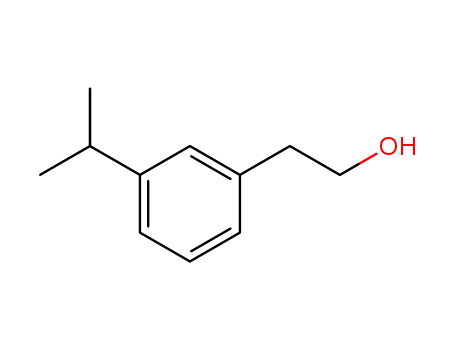
- Chemical Name:Benzeneethanol, 3-(1-methylethyl)-
- CAS No.:68480-22-8
- Molecular Formula:C11H16O
- Molecular Weight:164.247
- Hs Code.:
- European Community (EC) Number:270-899-4
- UNII:FY788PSU8J
- DSSTox Substance ID:DTXSID2071572
- Nikkaji Number:J326.647H
- Wikidata:Q81999260
- Mol file:68480-22-8.mol
Synonyms:68480-22-8;m-Isopropylphenethyl alcohol;Benzeneethanol, 3-(1-methylethyl)-;3-iso-Propylphenethyl alcohol;2-[3-(propan-2-yl)phenyl]ethan-1-ol;FY788PSU8J;3-(1-Methylethyl)benzeneethanol;EINECS 270-899-4;2-(3-(PROPAN-2-YL)PHENYL)ETHAN-1-OL;3-isopropyl-phenethyl alcohol;2-(3-propan-2-ylphenyl)ethanol;UNII-FY788PSU8J;SCHEMBL1241139;DTXSID2071572;2-(3-Isopropylphenyl)ethan-1-ol;AKOS006310345;CS-0273858;EN300-1636082