10.1016/j.bmc.2008.07.015
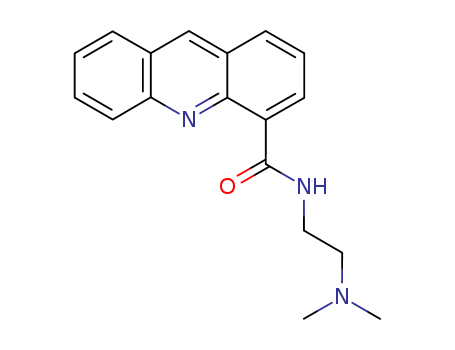
The research focuses on the design, synthesis, and preliminary biological evaluation of acridine compounds for a combined targeted chemo-radionuclide therapy approach to treat melanoma. The study exploits the structural similarity of these compounds to benzamides, which are known to have a specific affinity for melanin. The experiments involved the preparation of iodo-acridone and acridine carboxamides, which were evaluated for their in vitro cytotoxic properties and then radioiodinated with [125I]NaI for high specific activity. Biodistribution studies in B16F0 murine melanoma tumor-bearing mice were conducted to assess the tumor concentrations and in vivo kinetic profiles of the compounds. The main reactants used in the synthesis include various iodo-acridone and acridine carboxamide derivatives, along with reagents like [125I]NaI for radioiodination. Analyses included cytotoxicity assays, partition coefficient measurements, in vitro binding to melanin, and in vivo biodistribution studies to evaluate the compounds' affinity for melanoma tumors and their elimination rates. The research aimed to identify lead candidates for use in radionuclide therapy and/or chemotherapy of melanoma, with a focus on compounds that demonstrated high, long-lasting tumor concentrations and favorable kinetic profiles for targeted radionuclide therapy.