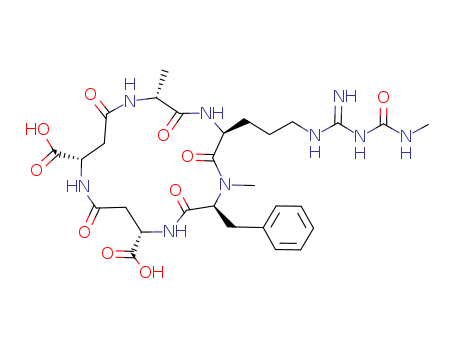
- Chemical Name:(2R,5S,8S,11S,15S)-5-[3-({(E)-amino[(methylcarbamoyl)amino]methylidene}amino)propyl]-8-benzyl-2,7-dimethyl-3,6,9,13,17-pentaoxo-1,4,7,10,14-pentaazacycloheptadecane-11,15-dicarboxylic acid
- CAS No.:243975-37-3
- Molecular Formula:C29H41N9O10
- Molecular Weight:675.699
- Hs Code.:
- Mol file:243975-37-3.mol
Synonyms:Argifin;FTD 0668