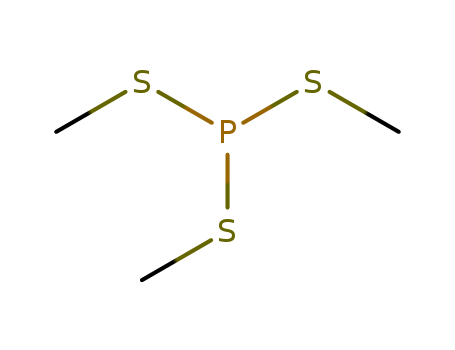
- Chemical Name:Phosphorotrithious acid, trimethyl ester
- CAS No.:816-80-8
- Molecular Formula:C3H9PS3
- Molecular Weight:172.276
- Hs Code.:
- DSSTox Substance ID:DTXSID60231217
- Nikkaji Number:J50.495E
- Wikidata:Q83112068
- Mol file:816-80-8.mol
Synonyms:Phosphorotrithious acid, trimethyl ester;Trimethyl phosphorotrithioate;816-80-8;trimethyl trithiophosphite;BRN 1697991;Tris(methylthio)phosphine;C3H9PS3;tris(methylsulfanyl)-phosphane;SCHEMBL10339205;DTXSID60231217;C3-H9-P-S3;LS-108992