10.1080/10426500008042091
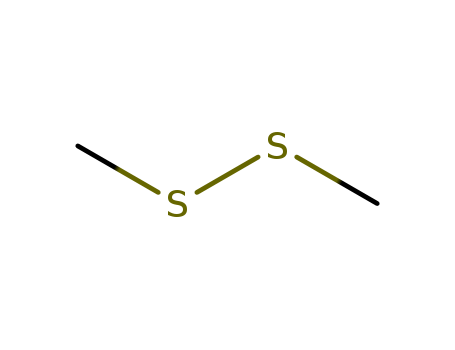
The research discusses a new method of decarboxylative sulfanylation of some phenylsulfonyl arylacetic acids. The purpose of this study was to investigate the reaction of α-phenylsulfonyl arylacetic acids with NaH in DMSO and dimethyl disulfide, leading to the formation of α-sulfanylated benzyl phenyl sulfones. The researchers found that while certain acids resulted in the corresponding sulfanylated sulfones, one acid yielded an unsulfanylated sulfone. The study also explored the mechanisms behind these reactions, suggesting that decarboxylation occurs at the initial stage, leading to the formation of α-sulfonyl carbanions which then undergo sulfanylation. Key chemicals used in the process include phenylacetic acid, 4-methoxyphenylacetic acid, α-phenylpropionic acid, NaH, DMSO, and dimethyl disulfide. The conclusions drawn from the study mark the first instance of α-phenylsulfonyl carbanions, generated by decarboxylation of the corresponding carboxylic acids, reacting with a sulfur electrophile.
10.1246/bcsj.51.2435
The study investigates the catalytic activities of salicylaldehyde derivatives, specifically focusing on the synthesis of 3-, 4-, and 5-dimethylsulfonio derivatives of salicylaldehyde. These derivatives were prepared from corresponding bromo-2-methoxybenzylidene dibromides, bromo-o-anisaldehyde diethyl acetals, methylthio-o-anisaldehydes, and (methylthio)salicylaldehydes through a series of chemical reactions involving reagents such as sodium ethoxide, dimethyl disulfide, and methyl p-toluenesulfonate. The study also examined the racemization of L-glutamic acid catalyzed by these salicylaldehyde derivatives in the presence of copper(I) ion at pH 10 and 80°C. The results indicated that the dimethylsulfonio derivatives exhibited higher catalytic activity than known salicylaldehyde derivatives, as evidenced by their larger Hammett's constant values.


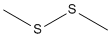
 Xn,
Xn, F,
F,  T+,
T+,  N
N


