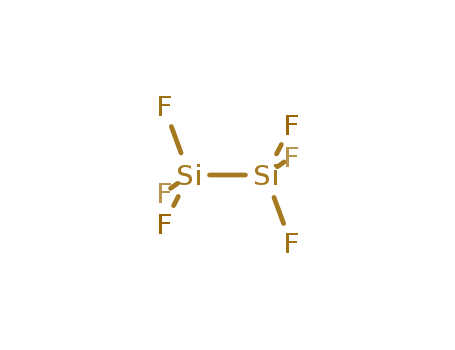- Chemical Name:Hexafluorodisilane
- CAS No.:13830-68-7
- Molecular Formula:F6Si2
- Molecular Weight:170.161
- Hs Code.:
- DSSTox Substance ID:DTXSID70160564
- Nikkaji Number:J627.961I
- Wikipedia:Hexafluorodisilane
- Wikidata:Q18211432
- Mol file:13830-68-7.mol
Synonyms:Hexafluorodisilane;13830-68-7;trifluoro(trifluorosilyl)silane;Si2F6;Hexafluordisilan;DTXSID70160564