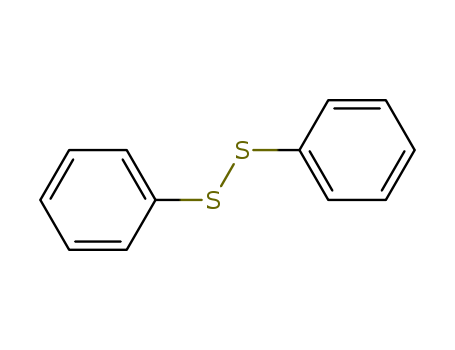
- Chemical Name:Diphenyl disulfide
- CAS No.:882-33-7
- Deprecated CAS:1071709-24-4,1195339-75-3,1195339-75-3
- Molecular Formula:C12H10S2
- Molecular Weight:218.343
- Hs Code.:29093090
- European Community (EC) Number:212-926-4
- NSC Number:2689
- UNII:7P54H519IJ
- DSSTox Substance ID:DTXSID6022131
- Nikkaji Number:J2.117B
- Wikipedia:Diphenyl_disulfide
- Wikidata:Q2423101
- Metabolomics Workbench ID:45132
- ChEMBL ID:CHEMBL462861
- Mol file:882-33-7.mol
Synonyms:diphenyl disulfide


 Xi
Xi


