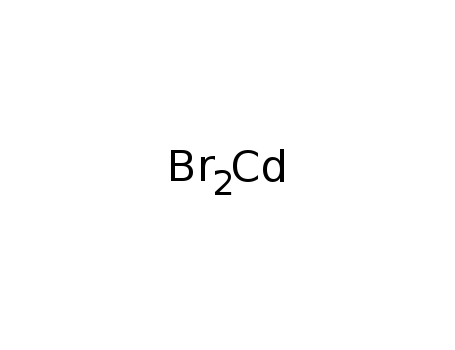- Chemical Name:Cadmium bromide
- CAS No.:7789-42-6
- Molecular Formula:Br2Cd
- Molecular Weight:272.218
- Hs Code.:
- European Community (EC) Number:232-165-1
- UN Number:2570
- UNII:7726AXS0WH
- DSSTox Substance ID:DTXSID30895027
- Nikkaji Number:J43.715H
- Wikipedia:Cadmium bromide,Cadmium_bromide
- Wikidata:Q417129
- Mol file:7789-42-6.mol
Synonyms:CADMIUM BROMIDE;CADMIUMBROMIDE;Br2Cd;Br2-Cd;CADMIUM BROMATUM;CMB (CHRIS Code);Bromuro de cadmio (cdbr2);CADMIUM BROMIDE [MI];CADMIUM BROMIDE [HSDB];CADMIUM BROMATUM [HPUS];CADMIUM BROMIDE [WHO-DD];DTXSID30895027;KPWJBEFBFLRCLH-UHFFFAOYSA-L;Methyl furan-3-yl(methyl)carbamate;AKOS015832993;Q417129