- Chemical Name:Aniline hydrochloride
- CAS No.:142-04-1
- Deprecated CAS:1415719-73-1
- Molecular Formula:C6H7N.HCl
- Molecular Weight:129.589
- Hs Code.:29214100
- European Community (EC) Number:306-925-9,205-519-8
- ICSC Number:1013
- NSC Number:7910
- UN Number:1548
- UNII:576R1193YL
- DSSTox Substance ID:DTXSID3020091
- Wikipedia:Anilinium_chloride
- Wikidata:Q2287905
- ChEMBL ID:CHEMBL3182415
- Mol file:142-04-1.mol
Synonyms:aniline;aniline aluminium salt;aniline dihydrofluoride;aniline diphosphate (1:1);aniline diphosphate (3:1);aniline diphosphate (4:1);aniline hydrobromide;aniline hydrochloride;aniline hydrochloride-(14)C-labeled cpd;aniline hydrochloride-(15)N-labeled cpd;aniline hydrofluoride;aniline hydrogen iodide;aniline monosulfate;aniline nitrate;aniline perchlorate;aniline phosphate (1:1);aniline phosphate (1:2);aniline phosphate (2:1);aniline phosphonate (1:1);aniline sulfate;aniline sulfate (2:1);aniline sulfate (2:1), (14)C-labeled cpd;aniline, (13)C-labeled cpd;aniline, (14)C-labeled cpd;aniline, 15N-labeled cpd;aniline, 2-(13)C-labeled cpd;aniline, 3-(13)C-labeled cpd;aniline, 3H-labeled cpd;aniline, 4-(13)C-labeled cpd;aniline, conjugate acid;aniline, ion(1+);aniline, magnesium (1:1) salt;aniline, monolithium salt;aniline, sodium salt


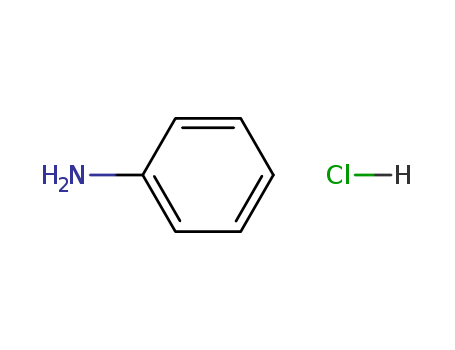
 T,
T,  N
N


