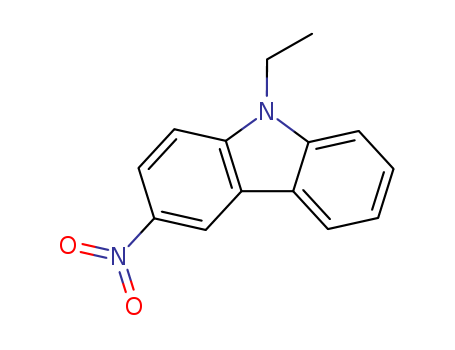
- Chemical Name:9-Ethyl-3-nitrocarbazole
- CAS No.:86-20-4
- Molecular Formula:C14H12N2O2
- Molecular Weight:240.261
- Hs Code.:2933990090
- European Community (EC) Number:201-655-7
- NSC Number:17817
- UNII:00X39KP0AS
- DSSTox Substance ID:DTXSID0044995
- Nikkaji Number:J36.856C
- Wikidata:Q27231385
- ChEMBL ID:CHEMBL1366879
- Mol file:86-20-4.mol
Synonyms:9-Ethyl-3-nitrocarbazole;86-20-4;9-Ethyl-3-nitro-9H-carbazole;3-Nitro-N-Ethyl carbazole;3-Nitro-N-ethylcarbazole;9H-Carbazole, 9-ethyl-3-nitro-;3-Nitro-9-ethylcarbazole;Carbazole, 9-ethyl-3-nitro-;n-Ethyl-3-Nitrocarbazole;UNII-00X39KP0AS;9-ethyl-3-nitro-9H-carbazol;00X39KP0AS;DTXSID0044995;EINECS 201-655-7;NSC 17817;NSC-17817;NSC17817;Piperilatehydrochloride;Oprea1_271225;MLS001018110;SCHEMBL149167;3-Nitro-9-ethyl-9H-carbazole;CHEMBL1366879;DTXCID8024995;6-NITRO-9-ETHYLCARBAZOLE;9-Ethyl-3-nitrocarbazole, 98%;HMS2640P13;9-Ethyl-3-nitro-9H-carbazole #;Tox21_301272;MFCD00022215;AKOS001030159;AKOS015916654;CCG-253850;CAS-86-20-4;NCGC00246000-01;NCGC00256185-01;AC-18965;AS-14545;SMR000354314;CS-0204377;FT-0631405;E85384;A841576;W-109312;Q27231385;Z56759381