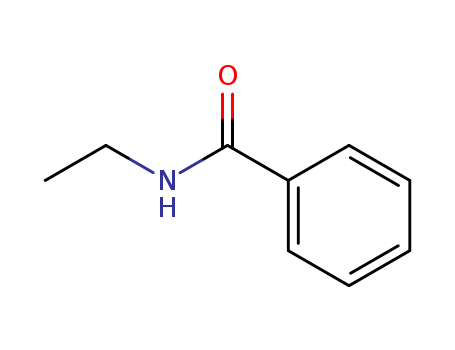
- Chemical Name:N-Ethylbenzamide
- CAS No.:614-17-5
- Molecular Formula:C9H11 N O
- Molecular Weight:149.192
- Hs Code.:2924299090
- European Community (EC) Number:664-988-0
- NSC Number:20558
- UNII:SW509B0V2A
- DSSTox Substance ID:DTXSID10210315
- Nikkaji Number:J54.488D
- Wikidata:Q72472771
- ChEMBL ID:CHEMBL500597
- Mol file:614-17-5.mol
Synonyms:N-ethylbenzamide