10.1080/00397910801929465
The study presents a convenient approach to the synthesis of dialkyl 5-oxo-1,2-dihydro-5H-chromeno[4,3-b]pyridine-2,3-dicarboxylates, which are compounds considered privileged scaffolds in synthetic and pharmacological research due to their potential pharmacological activities. The researchers focused on the chemical behavior of enaminocarbaldehydes derived from the coumarin moiety under intramolecular Wittig reaction conditions. Key chemicals used in the study include triphenylphosphine, dimethyl or diethyl acetylenedicarboxylates, and various enaminoaldehydes (1a–g), which are readily available by treating 4-hydroxycoumarin with different amines and then formylated under Vilsmeier-Haack reaction conditions. These chemicals served the purpose of facilitating the synthesis of the target compounds in good to high yields, expanding the scope of synthetic methods for creating biologically active ingredients and fluorescent dyes.
10.1016/j.tet.2009.02.051
The study focuses on the novel chemistry of β-carbolines, specifically exploring the synthesis of polycyclic scaffolds through various chemical reactions. β-carbolines are heterocyclic compounds found in numerous natural alkaloids with diverse biological activities, making them of interest for pharmaceutical applications. The researchers utilized ring-closing metathesis (RCM) and combined it with other cyclization processes such as Diels-Alder reactions to generate complex molecular structures in a single synthetic step. Chemicals used in the study include allyl-, vinyl-, ethynyl-, and propargyl-b-carbolines as substrates, as well as Grignard reagents and various catalysts like [Ru]-I, [Ru]-II, and [Ru]-III for metathesis reactions. Additionally, compounds like dimethyl acetylenedicarboxylate (DMAD) and dimethyl maleate were employed as dienophiles in Diels-Alder reactions. The purpose of these chemicals was to functionalize β-carbolines and construct more complex heterocycles, which could potentially lead to the development of new drugs with various therapeutic properties. The study also observed novel domino processes and rearrangements, such as Stevens rearrangement, providing insights into the complex polycyclic chemistry of β-carbolines.
10.1055/s-1997-1403
The research focuses on the synthesis of polysubstituted phenylphenols from substituted anilines, which are of interest due to their photochromic properties. The purpose of the study was to develop a general preparation method for 4-aryl-2,3-dicarboxyphenols derivatives, which are required for further elaboration. The researchers adopted a strategy starting with 2-arylfurans and coupling them with dimethyl acetylenedicarboxylate (DMAD), followed by the opening of the resulting adducts leading to the biaryls. The process involved a Diels-Alder cycloaddition, which was accelerated by the addition of a Lewis acid, specifically ZnI2. The study concluded that satisfactory yields of [4+2] adducts were obtained in most cases, and the β-elimination step leading to substituted phenylphenols was found to be immediate and spontaneous when an electron-releasing group was present on the aryl group. However, the presence of an electron-withdrawing group limited the β-elimination, suggesting that electronic factors and the conformation of the aryl-oxygen bridge system play a role in the stability of the intermediates. The chemicals used in the process included various substituted anilines to synthesize arylfurans, DMAD as the dienophile, and ZnI2 as the Lewis acid catalyst.
10.1039/b821179e
The research focuses on the stoichiometric transformations of 2,6-xylenol via sp3 C–H bond cleavage reactions using transition-metal complexes, specifically ruthenium. The purpose of the study is to explore the potential for direct functionalization of organic molecules through carbon-hydrogen bond cleavage reactions. The researchers achieved the insertion of dimethyl acetylenedicarboxylate (DMAD) into the Ru–C bond of a cycloruthenated complex, leading to the formation of a seven-membered oxaruthenacycle. This complex exhibited fluxional behavior in solution, and upon heating, a 1,3-H shift reaction occurred, resulting in an h3-allylic complex. Acidolysis and iodolysis of the complexes produced various phenolic compounds and benzopyrans, respectively.
10.1016/0040-4020(80)85037-X
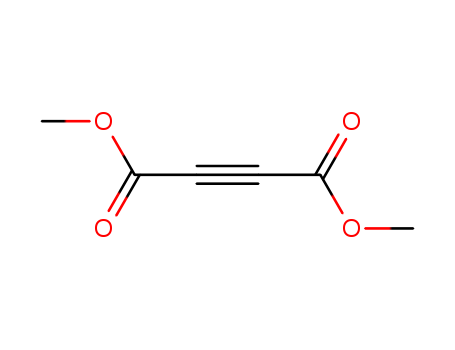
The study focuses on the synthesis and reactions of various chemical compounds, primarily involving the transformation of different isomers and the creation of new compounds through specific chemical reactions. Key chemicals include tetrachloride (TCE), which is used in cycloaddition reactions with dienes like 5 to form adducts such as 16 and 17. The study explores the reactivity and selectivity of these compounds under different conditions, such as varying solvents and the presence of bases like CsF. Other chemicals like dimethyl acetylenedicarboxylate (DAD) and dimethyl fumarate are also used in cycloaddition reactions with 5, yielding different products like 19 and 22. The study investigates the influence of steric and electronic factors on the reactivity and selectivity of these reactions, utilizing techniques like NMR and IR spectroscopy to analyze the structures and properties of the synthesized compounds.
10.1055/s-2000-8212
The research investigates the addition of zwitterionic intermediates, generated by triphenylphosphane and dimethyl acetylenedicarboxylate (DMAD), to 1,2- and 1,4-benzoquinones to synthesize novel unsaturated ?-spirolactones. The purpose is to explore the reactivity of quinones towards these intermediates and develop a method for creating highly functionalized spirolactones. The study finds that both ortho- and para-quinones readily react with the zwitterionic intermediate, yielding spirolactones in moderate to high yields. Key chemicals used include triphenylphosphane, DMAD, various benzoquinones (such as 4,6-di-tert-butyl-3-methoxy-1,2-benzoquinone and 1,4-benzoquinone), and solvents like benzene. The results show that this method provides a facile route to produce spirolactones, which are present in several biologically active natural products.
10.1002/chem.202001639
The study presents an innovative approach to the enantioselective generation of quaternary carbon stereocenters through the addition of substituted cyanoacetate esters to acetylenic esters, utilizing a chiral enantiopure cobalt(III) complex as a hydrogen bond donor catalyst. The research successfully identifies the cobalt complex Δ-[Co((S,S)-dpen)3]3+ 2Cl–B(C6F5)4– (where dpen = 1,2-diphenylethylenediamine) as an effective catalyst for this reaction, achieving high enantioselectivities (up to 98% ee) and Z/E selectivities (up to 99:1) under optimal conditions. The study also explores the scope of the reaction with various substrates, revealing the catalyst's versatility and its potential for synthetic applications. Additionally, the research delves into the mechanism of the reaction, suggesting that hydrogen bonding between the catalyst's NH groups and the substrates plays a crucial role in the catalytic process. This work significantly advances the field of enantioselective catalysis, providing a new tool for the synthesis of complex molecular structures with quaternary carbon stereocenters.
10.1007/BF00478853
The research focuses on the synthesis and properties of 2-diazomethyl-4-furanones, which are cyclic vinylogs of diazo ketones. The study aims to investigate the acidic transformations of bisdiazo ketones, specifically looking at the retention of one diazo group and the possibility of synthesizing 2-diazomethyl-4-furanones from them. The researchers synthesized 1,5-bisdiazo-3-alkylpentane-2,4-diones by reacting alkyl-malonic acid chlorides with diazomethane. These compounds readily undergo intramolecular cyclization under acidic conditions to form 2-diazomethyl-3-alkyl-4-furanones. The study concludes that diazomethyl-4-furanones exhibit reactions characteristic of aliphatic diazo compounds and that their structure was confirmed through X-ray diffraction analysis. The chemicals used in the process include diazomethane, alkyl-malonic acid chlorides, and various reagents such as hydrogen chloride and dimethyl acetylenedicarboxylate.


 C
C


