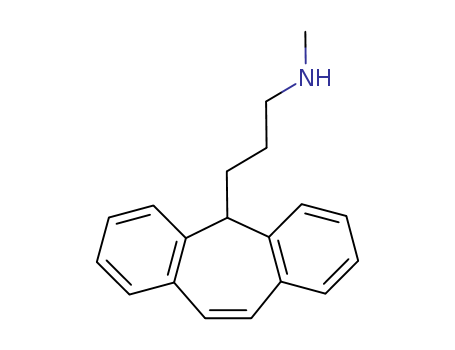
- Chemical Name:Protriptyline
- CAS No.:438-60-8
- Molecular Formula:C19H21 N
- Molecular Weight:263.382
- Hs Code.:2921499090
- European Community (EC) Number:207-119-9
- UNII:4NDU154T12
- DSSTox Substance ID:DTXSID0023535
- Nikkaji Number:J5.739H
- Wikipedia:Protriptyline
- Wikidata:Q408432
- NCI Thesaurus Code:C61913
- RXCUI:8886
- Pharos Ligand ID:DKSR4U8163BL
- Metabolomics Workbench ID:42721
- ChEMBL ID:CHEMBL668
- Mol file:438-60-8.mol
Synonyms:Hydrochloride, Protriptyline;Protriptyline;Protriptyline Hydrochloride;Vivactil