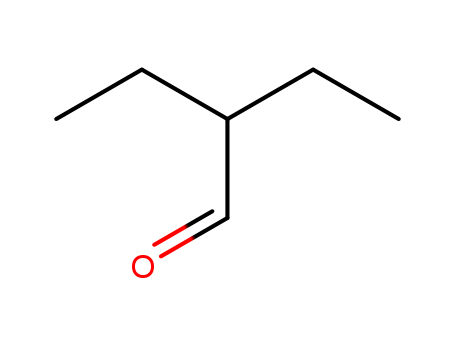
- Chemical Name:2-Ethylbutanal
- CAS No.:97-96-1
- Molecular Formula:C6H12O
- Molecular Weight:100.161
- Hs Code.:29121900
- European Community (EC) Number:202-623-5
- NSC Number:6757
- UN Number:1178
- UNII:676JY5569P
- DSSTox Substance ID:DTXSID3049380
- Nikkaji Number:J35.112A
- Wikidata:Q27264080
- Metabolomics Workbench ID:44761
- ChEMBL ID:CHEMBL273782
- Mol file:97-96-1.mol
Synonyms:2-Ethylbutanal;2-ETHYLBUTYRALDEHYDE;97-96-1;Butanal, 2-ethyl-;Diethylacetaldehyde;3-Formylpentane;2-Ethylbutyric aldehyde;Butyraldehyde, 2-ethyl-;2-EthYl-Butanal;alpha-Ethylbutanal;2-Ethylbutyric aledhyde;Ethyl butyraldehyde;Aldehyde 2-ethylbutyrique;Diethyl acetaldehyde;2-Ethyl-butyraldehyde;alpha-Ethylbutyraldehyde;Ethylbutyraldehyde;FEMA No. 2426;NSC 6757;2-ethyl butyraldehyde;Aldehyde 2-ethylbutyrique [French];EINECS 202-623-5;UN1178;.alpha.-Ethylbutyraldehyde;BRN 1209330;(C2H5)2CHCHO;CHEMBL273782;DTXSID3049380;UNII-676JY5569P;NSC-6757;676JY5569P;2-Ethylbutyraldehyde [UN1178] [Flammable liquid];Butanal, 2-etil-;.alpha.-Ethylbutanal;racemic 2-ethylbutanal;2-Ethylbutyraldehyde, 8CI;SCHEMBL29715;2-Ethylbutyraldehyde, >=92%;DTXCID6029339;WLN: VHY2 & 2;FEMA 2426;NSC6757;CHEBI:173345;2-ETHYLBUTYRALDEHYDE [FCC];2-ETHYLBUTYRALDEHYDE [FHFI];STR03920;Tox21_202883;BDBM50028843;MFCD00006985;NA1178;AKOS000120285;LS-2714;UN 1178;CAS-97-96-1;(2-chlorophenyl)-(1-piperidyl)methanone;NCGC00260429-01;E0069;FT-0709910;EN300-20417;2-Ethylbutyraldehyde [UN1178] [Flammable liquid];Q-101277;Q27264080;F2190-0625


 F,
F,  Xi
Xi


