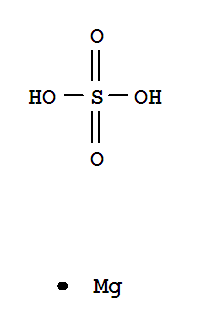
- Chemical Name:Magnesium Sulfate
- CAS No.:7487-88-9
- Deprecated CAS:139939-75-6,849607-35-8,849607-35-8
- Molecular Formula:MgSO4
- Molecular Weight:120.369
- Hs Code.:2833210000
- European Community (EC) Number:231-298-2,242-691-3,686-508-9
- ICSC Number:1197
- UNII:ML30MJ2U7I
- DSSTox Substance ID:DTXSID6042105
- Nikkaji Number:J3.103H
- Wikipedia:Magnesium sulfate,Magnesium_sulfate
- Wikidata:Q288266
- NCI Thesaurus Code:C623,C66051
- RXCUI:6585,1311625
- ChEMBL ID:CHEMBL2021423
- Mol file:7487-88-9.mol
Synonyms:Heptahydrate Magnesium Sulfate;Magnesium Sulfate;Magnesium Sulfate, Heptahydrate;Sulfate, Magnesium


 Xi
Xi


