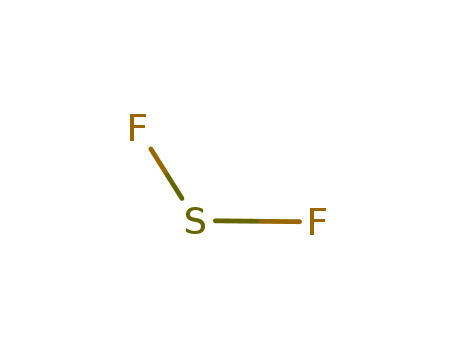
- Chemical Name:Sulfur difluoride
- CAS No.:13814-25-0
- Molecular Formula:F2S
- Molecular Weight:70.0628
- Hs Code.:
- DSSTox Substance ID:DTXSID30160500
- Nikkaji Number:J134.313K
- Wikipedia:Sulfur difluoride
- Wikidata:Q553164
- Mol file:13814-25-0.mol
Synonyms:SF2 compound;sulfur difluoride;sulfur fluoride