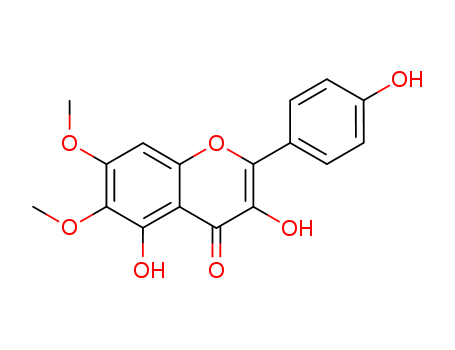
- Chemical Name:Eupalitin
- CAS No.:29536-41-2
- Molecular Formula:C17H14 O7
- Molecular Weight:330.294
- Hs Code.:2914509090
- UNII:P5KF23690D
- DSSTox Substance ID:DTXSID20183723
- Nikkaji Number:J21.597J
- Wikipedia:Eupalitin
- Wikidata:Q10859709
- Metabolomics Workbench ID:25953
- Mol file:29536-41-2.mol
Synonyms:3,5,4'-trihydroxy-6,7-dimethoxyflavone