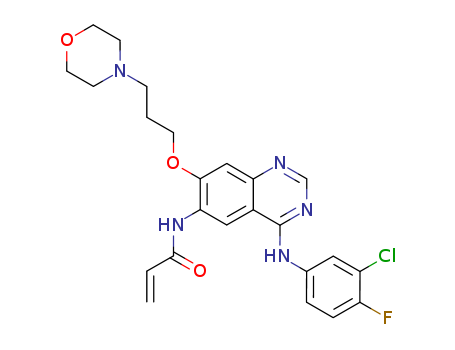
10.1021/jm400349k
This research presents the repurposing of kinase scaffolds for the discovery of new drugs to combat neglected tropical diseases, specifically focusing on human African trypanosomiasis (HAT), a disease caused by the protozoan parasite Trypanosoma brucei. The study aims to expedite drug discovery by utilizing chemical scaffolds from drugs approved for other indications, demonstrating the potential of lapatinib and canertinib, two 4-anilinoquinazolines with low micromolar EC50 values against T. brucei. The researchers synthesized and tested several potent 4-anilinoquinazolines, leading to the identification of NEU617 (23a), a highly potent and orally bioavailable inhibitor of trypanosome replication. The compound 23a was found to block kinetoplast duplication and arrest cytokinesis in trypanosomes, offering a new chemical tool for studying the regulation of the trypanosome cell cycle. The study concludes that compounds based on established human EGFR inhibitor chemotypes can be effective against HAT and that further optimization of these chemotypes is necessary to improve their pharmacokinetic properties and effectiveness in treating HAT. The chemicals used in the process include a series of 4-anilinoquinazoline derivatives, with lapatinib (GW572016, 1) and canertinib (CI-1033) as the starting points for optimization.