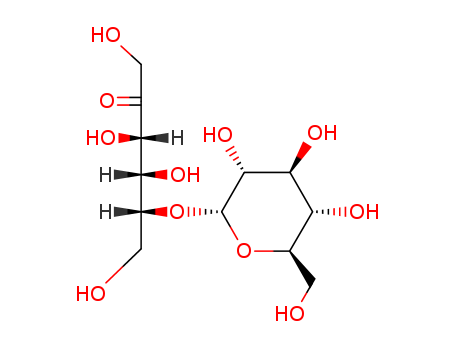
- Chemical Name:D-LEUCROSE
- CAS No.:7158-70-5
- Molecular Formula:C12H22O11
- Molecular Weight:342.3
- Hs Code.:29329990
- Mol file:7158-70-5.mol
Synonyms:MALT SUGAR;MALATOSE,D;starch sugar;MALTOSE H2O;Corn sweetener;D-MALATOSE;starch syrup;D-(+)-maltose;maltose monohydrate;