10.1248/cpb.c16-00190
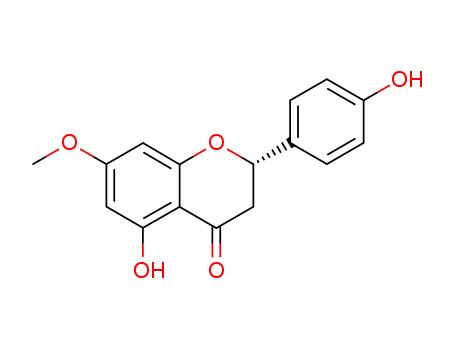
The research presents a simple synthesis of sakuranetin and selinone, two biologically active compounds, utilizing the regioselective deacetylation of naringenin triacetate. Imidazole was used to selectively deacetylate naringenin triacetate at the C-7 position, leading to the formation of an intermediate that was subsequently methylated to produce sakuranetin. For the synthesis of selinone, the same starting material was subjected to transesterification with 2-propanol in the presence of Candida antarctica lipase B, resulting in selective deacetylation at the C-4′ position. This intermediate was then prenylated under Mitsunobu conditions to yield selinone. The study highlights the importance of these chemicals in achieving the desired regioselectivity and in the overall synthesis of these bioactive compounds.