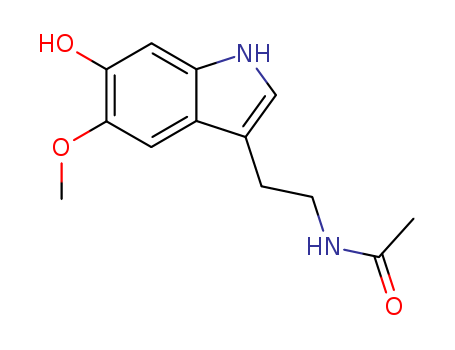
- Chemical Name:6-Hydroxymelatonin
- CAS No.:2208-41-5
- Molecular Formula:C13H16N2O3
- Molecular Weight:248.282
- Hs Code.:2933990090
- European Community (EC) Number:636-648-1
- UNII:TV437T5077
- DSSTox Substance ID:DTXSID00176577
- Nikkaji Number:J102.947I
- Wikipedia:6-Hydroxymelatonin
- Wikidata:Q20707319
- Pharos Ligand ID:STNG3MMZX2NH
- Metabolomics Workbench ID:38459
- ChEMBL ID:CHEMBL127421
- Mol file:2208-41-5.mol
Synonyms:6-hydroxymelatonin;6-oxymelatonin