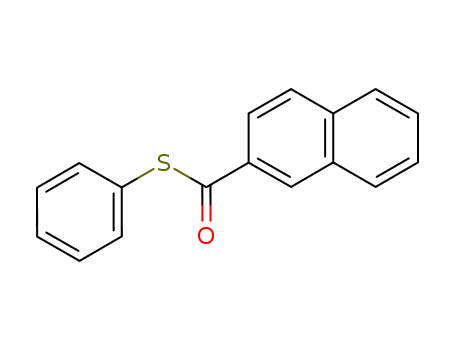
- Chemical Name:S-Phenyl 2-naphthalenethiocarboxylate
- CAS No.:28118-49-2
- Molecular Formula:C17H12 O S
- Molecular Weight:264.34
- Hs Code.:
- DSSTox Substance ID:DTXSID80182393
- Nikkaji Number:J42.446C
- Wikidata:Q83053067
- Mol file:28118-49-2.mol
Synonyms:S-Phenyl 2-naphthalenethiocarboxylate;28118-49-2;2-NAPHTHOIC ACID, S-PHENYL ESTER;2-Naphthalenecarboxylic acid, thio-, S-phenyl ester;S-phenyl naphthalene-2-carbothioate;C16H12OS;DTXSID80182393;C16-H12-O-S;2-Naphthoic acid, thio-, S-phenyl ester;LS-95390;2-Naphthalene(thiocarboxylic acid)S-phenyl ester