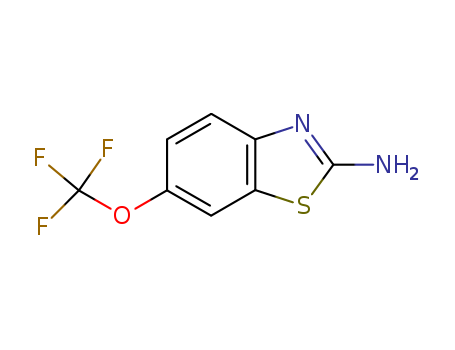
- Chemical Name:Riluzole
- CAS No.:1744-22-5
- Molecular Formula:C8H5F3N2OS
- Molecular Weight:234.202
- Hs Code.:29342000
- European Community (EC) Number:605-724-6
- NSC Number:759823,753433
- UNII:7LJ087RS6F
- DSSTox Substance ID:DTXSID3045192
- Nikkaji Number:J22.921K
- Wikipedia:Riluzole
- Wikidata:Q415744
- NCI Thesaurus Code:C47704
- RXCUI:35623
- Pharos Ligand ID:CTLUL57W2GUZ
- Metabolomics Workbench ID:43044
- ChEMBL ID:CHEMBL744
- Mol file:1744-22-5.mol
Synonyms:2 Amino 6 trifluoromethoxybenzothiazole;2-Amino-6-trifluoromethoxybenzothiazole;PK 26124;PK-26124;PK26124;Rilutek;Riluzole;RP 54274;RP-54274;RP54274


 T,
T, Xi
Xi


