10.1021/ja01078a038
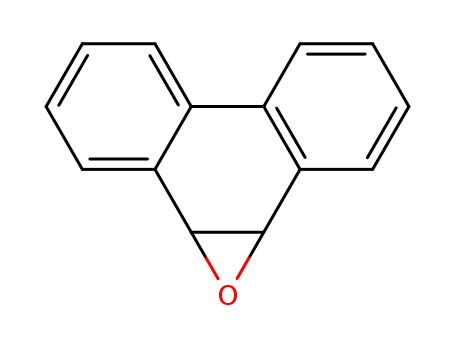
The study explores various chemical reactions and syntheses involving different compounds. One section focuses on the formolysis of alkyl p-nitrobenzenesulfonates, where different substrates like 6-methyl-5-heptenyl p-nitrobenzenesulfonate and 6-methyl-6-heptenyl p-nitrobenzenesulfonate are used to produce various alcohols and olefins. The yields and extents of reactions are analyzed and reported. Another part of the study describes the synthesis of epoxides of aromatic hydrocarbons, such as 9,10-dihydro-9,10-epoxyphenanthrene, 3,4-dihydro-3,4-epoxy-1,2-benzanthracene, and 3,4-dihydro-3,4-epoxy-10-methyl-1,2-benzanthracene, using trisdimethylaminophosphine and dialdehydes. These epoxides are sensitive to acid treatment and rearrange to form phenolic compounds. The study also involves the preparation of 4-bromo- and 4-iodo-2,5,7-trinitrofluorenones as reagents for forming charge-transfer complexes suitable for X-ray analysis. Various complexes are listed, and the study aims to explore the complex-forming abilities of these reagents and their potential use in X-ray crystallographic studies.