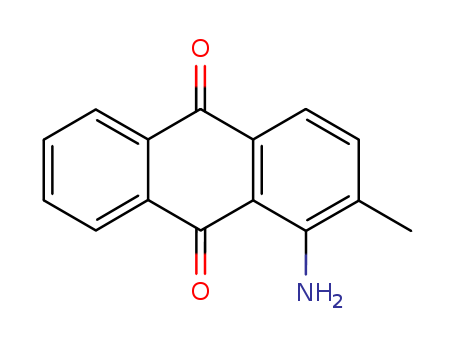
- Chemical Name:1-Amino-2-methylanthraquinone
- CAS No.:82-28-0
- Deprecated CAS:507484-51-7
- Molecular Formula:C15H11NO2
- Molecular Weight:237.258
- Hs Code.:2922399090
- European Community (EC) Number:201-408-3
- NSC Number:667744,39943
- UNII:RHN157F30Q
- DSSTox Substance ID:DTXSID7020057
- Nikkaji Number:J4.610H
- Wikidata:Q27155911
- NCI Thesaurus Code:C44302
- ChEMBL ID:CHEMBL1413787
- Mol file:82-28-0.mol
Synonyms:1-Amino-2-methyl-9,10-anthraquinone;1-amino-2-methylanthraquinone;disperse orange 11