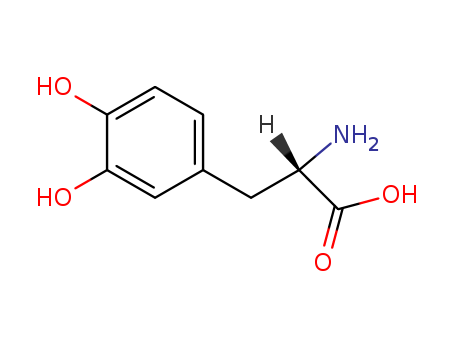
- Chemical Name:(2S)-2-azaniumyl-3-(3,4-dihydroxyphenyl)propanoate
- CAS No.:59-92-7
- Molecular Formula:C9H11NO4
- Molecular Weight:197.191
- Hs Code.:29225090
- Mol file:59-92-7.mol
Synonyms:(2S)-2-azaniumyl-3-(3,4-dihydroxyphenyl)propanoate;L-dihydroxy-phenylalanine;L-dopa zwitterion;(2S)-2-ammonio-3-(3,4-dihydroxyphenyl)propanoate;CHEBI:57504


 Xn
Xn

