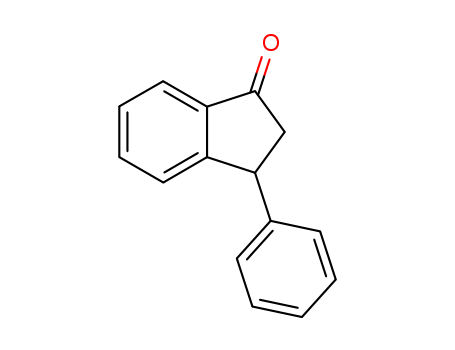
- Chemical Name:3-Phenyl-1-indanone
- CAS No.:16618-72-7
- Molecular Formula:C15H12O
- Molecular Weight:208.26
- Hs Code.:2914399090
- European Community (EC) Number:625-438-5
- NSC Number:82364
- DSSTox Substance ID:DTXSID701314027
- Nikkaji Number:J36.988H
- ChEMBL ID:CHEMBL1571712
- Mol file:16618-72-7.mol
Synonyms:3-Phenyl-1-indanone;16618-72-7;3-Phenyl-2,3-dihydro-1H-inden-1-one;1-Indanone, 3-phenyl-;1H-Inden-1-one, 2,3-dihydro-3-phenyl-;3-Phenylindan-1-one;3-Phenylindanone;3-phenyl-2,3-dihydroinden-1-one;NSC 82364;BRN 1370263;4-07-00-01673 (Beilstein Handbook Reference);NSC82364;MLS000691949;SCHEMBL181713;3-Phenyl-1-indanone, 98%;WLN: L56 BVT&J DR;(+/-)-3-Phenyl-1-indanone;CHEMBL1571712;DTXSID701314027;HMS2629C20;AMY13690;3-phenyl-2,3-dihydro-inden-1-one;MFCD00037722;NSC-82364;AKOS004119419;AKOS016038080;1H-Inden-1-one,3-dihydro-3-phenyl-;NCGC00246563-01;LS-81336;SMR000333984;1H-Indene-1-one, 2,3-dihydro-3-phenyl-;CS-0315124;FT-0616326;9K-043;I10111;A810702;J-010263


 Xn
Xn


