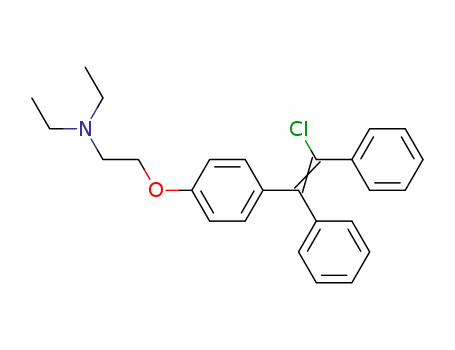
- Chemical Name:Clomiphene
- CAS No.:911-45-5
- Deprecated CAS:96189-16-1
- Molecular Formula:C26H28ClNO
- Molecular Weight:405.967
- Hs Code.:
- European Community (EC) Number:213-008-6
- UNII:R6D2UI4FLS
- DSSTox Substance ID:DTXSID201318048
- Wikipedia:Clomifene,Enclomifene
- Wikidata:Q28208734
- NCI Thesaurus Code:C28211
- RXCUI:2596
- Pharos Ligand ID:JCJQH2T9V16W,JCJR52WLRN2J
- Metabolomics Workbench ID:43157,149942
- ChEMBL ID:CHEMBL954
- Mol file:911-45-5.mol
Synonyms:Chloramiphene;Citrate, Clomiphene;Clomid;Clomide;Clomifen;Clomifene;Clomiphene;Clomiphene Citrate;Clomiphene Hydrochloride;Clostilbegit;Dyneric;Gravosan;Hydrochloride, Clomiphene;Klostilbegit;Serophene