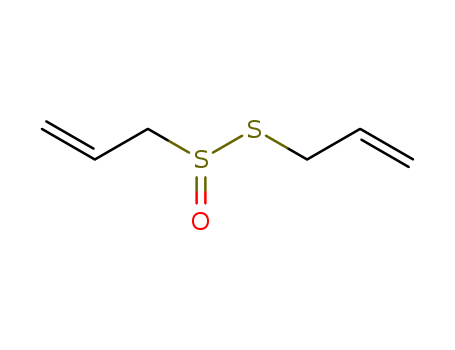
- Chemical Name:Allicin
- CAS No.:539-86-6
- Molecular Formula:C6H10OS2
- Molecular Weight:162.277
- Hs Code.:29329990
- European Community (EC) Number:208-727-7
- NSC Number:707388
- UNII:3C39BY17Y6
- DSSTox Substance ID:DTXSID6043707
- Nikkaji Number:J71.032F,J816.011B
- Wikipedia:Allicin
- Wikidata:Q409641,Q105125581
- NCI Thesaurus Code:C68521
- Pharos Ligand ID:RYB4QSHURHCL,RYBBG8YPK9FL
- Metabolomics Workbench ID:46312
- ChEMBL ID:CHEMBL359965
- Mol file:539-86-6.mol
Synonyms:allicin;allimin;allylthiosulfinate;allylthiosulphinic acid allyl ester;diallyl disulfide-oxide;thio-2-propene-1-sulfinic acid S-allyl ester