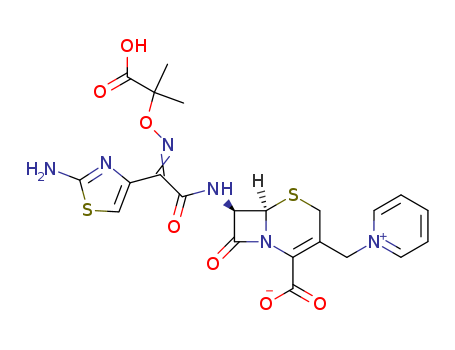
- Chemical Name:Ceftazidimum
- CAS No.:72558-82-8
- Molecular Formula:C22H22N6O7S2
- Molecular Weight:546.585
- Hs Code.:29419000
- European Community (EC) Number:276-715-9,616-626-8
- Wikipedia:Ceftazidime
- NCI Thesaurus Code:C66868,C90827
- RXCUI:2191,1545984
- Mol file:72558-82-8.mol
Synonyms:Ceftazidime;Ceftazidime Anhydrous;Ceftazidime Pentahydrate;Fortaz;Fortum;GR 20263;GR-20263;GR20263;LY 139381;LY-139381;LY139381;Pyridinium, 1-((7-(((2-amino-4-thiazolyl)((1-carboxy-1-methylethoxy)imino)acetyl)amino)-2-carboxy-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-en-3-yl)methyl)-, inner salt, pentahydrate, (6R-(6alpha,7beta(Z)))-;Tazidime


 Xn
Xn


