10.1002/anie.201004593
The study presents an innovative approach to C-C bond formation through NHC-catalyzed Michael addition to α,β-unsaturated aldehydes, utilizing redox activation. The researchers, Suman De Sarkar and Armido Studer, explore the use of N-heterocyclic carbenes (NHCs) to activate α,β-unsaturated aldehydes, which then react with various 1,3-dicarbonyl compounds to form dihydropyranones. They demonstrate that this method is effective with different nucleophiles and enals, achieving high yields and selectivity under mild conditions. The process involves a two-step umpolung reaction at the β-position of the α,β-unsaturated aldehyde, followed by a redox-type activation. The study also includes control experiments to rule out alternative mechanisms, such as kinetic O-acylation, and provides a proposed catalytic cycle for the process. This work contributes to the field of organocatalysis by offering a new strategy for conjugate addition reactions using soft C-nucleophiles and showcases the potential of NHCs in redox activation.
10.1016/j.tet.2007.11.036
The study presents a two-step synthesis method for benzo-fused 2,8-dioxabicyclo[3.3.1]nonane derivatives, utilizing a domino Knoevenagel condensation/intramolecular hetero-Diels-Alder reaction sequence. The process involves an initial intermolecular Knoevenagel condensation of a compound with active methylene compounds to form a heterodiene, which then undergoes intramolecular hetero-Diels-Alder cycloaddition. The research successfully optimized the reaction conditions, including the choice of catalyst and solvent, to achieve high yields of the desired products. The method demonstrates a novel route for constructing complex heterocycles with potential applications in medicinal chemistry.
10.1007/s11172-007-0243-5
The research presents a novel method for synthesizing 6-R-3-arylazo-1H-pyridazin-4-ones from difluoroboron chelates of 1,3-diketones. The study is based on the reactivity of the methyl group in these chelates with two equivalents of a diazonium salt. The synthesis involves a two-step process: first, the reaction of difluoroboron complexes of ?-diketones with diazonium salts at low temperatures to form dark red crystalline intermediates, followed by their decomposition upon refluxing in a pyridine-butanol mixture to yield the final 3-arylazo-1H-pyridazin-4-one derivatives. Acetylacetone and aroylacetones as the starting 1,3-diketones. Boron trifluoride etherate and tributyl borate for the preparation of difluoroboron chelates. The researchers utilized various spectroscopic techniques, including 1H and 13C NMR, IR, and mass spectrometry, to identify and analyze the structures of the synthesized compounds. The study also explored the possibility of structural isomers and confirmed the formation of the desired compounds through 2D NMR spectroscopy.
10.1016/j.tet.2016.10.037
The research focuses on the organocatalytic enantioselective synthesis of C3 functionalized indole derivatives, which are important for their potential bioactivity and use in therapeutic agents. The study explores the Michael addition reaction of indolylnitroalkenes with 1,3-dicarbonyl compounds, utilizing various chiral organocatalysts derived from Cinchona alkaloids. The reaction involves N-protected indolylnitroalkenes and acetylacetone as reactants, with the catalyst playing a crucial role in enantioselectivity. The experiments were optimized to achieve high yields and enantioselectivities, with the best results obtained using BnCPN as a catalyst in tetrahydrofuran (THF) as a solvent. The products were analyzed using techniques like chiral HPLC, NMR spectroscopy, and HRMS to determine their yields, enantiomeric excess (ee), and structures. The research also includes computational studies using DFT calculations to predict the transition state structures and understand the stereoselectivity of the reaction.
10.3184/030823409X12615671424822
The study investigates the use of L-arginine as a cost-effective and recyclable catalyst for the synthesis of α,β-unsaturated nitriles and ketones through the Knoevenagel condensation reaction in an ionic liquid medium. The chemicals used include aromatic, heteroaromatic, and α,β-unsaturated aldehydes, which react with malononitrile and acetylacetone to produce the desired nitriles and ketones. The ionic liquid, 1-ethyl-3-methylimidazolium ethylsulfate, serves as a green and recyclable reaction medium, while L-arginine acts as the catalyst, providing moderate to excellent yields (45–100%) and being successfully recycled for five runs without significant loss of activity. This approach offers a green and facile method for the synthesis of these important fine chemical industry products, which have applications as pre-polymers, antihypertensive agents, and calcium antagonists.
10.1016/j.tetasy.2008.09.030
The research investigates the development of a new and easy synthesis of chiral bifunctional organic catalysts obtained by combining (S)-t-leucine derivatives with (1R,2R)-trans-1,2-diamino-cyclohexane. Acetylacetone (also known as 2,4-pentanedione) is a β-diketone with the molecular formula C5H8O2. It is a colorless liquid with a characteristic odor and is commonly used as a ligand in coordination chemistry and as a precursor in organic synthesis. In this study, acetylacetone serves as the nucleophile in the model reaction. Nitrostyrene is an aromatic compound with a nitro group attached to a styrene backbone. The specific isomer used in this research is trans-b-nitrostyrene, which has the nitro group and the vinyl group in a trans configuration. The molecular formula for nitrostyrene is C8H7NO2. In the context of this study, nitrostyrene acts as the electrophile in the model reaction.
10.1021/om900283s
The study explores a novel ligand design for ?-diketiminato ligands to enhance the thermal stability of organoscandium complexes. The researchers introduced a "remote steric bulk" strategy, relocating the bulky groups from the ortho to the meta positions on the N-aryl substituents and increasing their size. This approach aims to stabilize low-coordinate organoscandium complexes by preventing metalation pathways that typically lead to decomposition. The study involves the synthesis of new ligands (1 and 2) using 2,4-pentanedione and specific anilines. These ligands were then used to form dialkyl scandium complexes (3 and 4) through reactions with scandium tris-alkyls. The resulting complexes exhibited significant improvements in thermal stability, with no signs of decomposition even when heated to over 100°C. The study also explored the reactivity of these complexes with activating reagents like [HNMe2Ph][B(C6F5)4] and [CPh3][B(C6F5)4], forming new cationic complexes (5 and 6) that retained remarkable thermal stability. These findings suggest that the redesigned ?-diketiminato ligands offer a promising strategy for stabilizing low-coordinate compounds of early transition and main group metals.


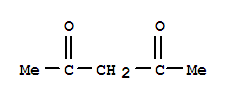
 Xn,
Xn, Xi
Xi


