- Chemical Name:Dibutylamine
- CAS No.:111-92-2
- Deprecated CAS:1357848-56-6
- Molecular Formula:C8H19N
- Molecular Weight:129.246
- Hs Code.:2921.19
- European Community (EC) Number:203-921-8
- ICSC Number:1337
- UN Number:2248
- UNII:2194M2LA21
- DSSTox Substance ID:DTXSID7024952
- Nikkaji Number:J2.886J
- Wikipedia:Dibutylamine
- Wikidata:Q1209336
- Metabolomics Workbench ID:130629
- ChEMBL ID:CHEMBL3184528
- Mol file:111-92-2.mol
Synonyms:di-n-butylamine;dibutylamine;dibutylamine hydrochloride;dibutylamine phosphate (3:1)


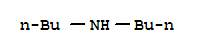
 Xn
Xn
 Xn:Harmful;
Xn:Harmful;

