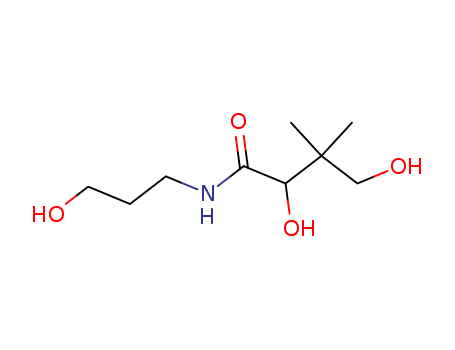
- Chemical Name:Panthenol
- CAS No.:16485-10-2
- Deprecated CAS:62507-76-0
- Molecular Formula:C9H19NO4
- Molecular Weight:205.254
- Hs Code.:29362400
- European Community (EC) Number:240-540-6
- NSC Number:759899,759127,302962
- UNII:WV9CM0O67Z
- DSSTox Substance ID:DTXSID3044598
- Nikkaji Number:J321.113D
- Wikipedia:Panthenol
- Wikidata:Q196473
- NCI Thesaurus Code:C82290
- RXCUI:1044977
- Metabolomics Workbench ID:123387
- ChEMBL ID:CHEMBL1371937
- Mol file:16485-10-2.mol
Synonyms:(+)-2,4-dihydroxy-N-(3-hydroxypropyl)-3,3-dimethylbutyramide;2,4-dihydroxy-N-(3-hydroxypropyl)-3,3-dimethylbutanamide;Bepanthen;butanamide, 2,4-dihydroxy-n-(3-hydroxypropyl)-3,3-dimethyl-, (+--)-;Corneregel;D-panthenol;dexpanthenol;Dexpanthenol Heumann;DL-panthenol;Ilopan;Marolderm;NasenSpray ratiopharm Panthenol;Nasicur;Otriven Dexpanthenol;Pan Rhinol;Pan-Ophtal;panthenol;Panthenol Braun;Panthenol Jenapharm;Panthenol LAW;Panthenol Lichtenstein;panthenol von ct;Panthenol-ratiopharm;Panthoderm;Panthogenat;pantothenol;Repa-Ophtal;Rhinoclir;Siozwo SANA;Ucee D;Urupan;Wund- und Heilsalbe LAW


 Xn
Xn


