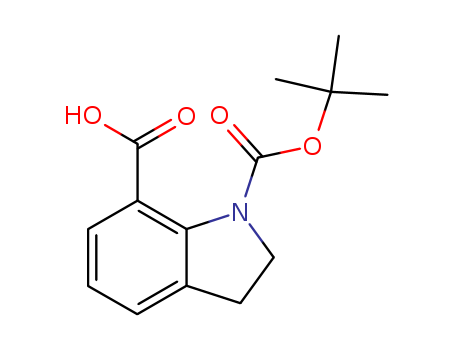
- Chemical Name:N-Boc-indoline-7-carboxylic acid
- CAS No.:143262-20-8
- Molecular Formula:C14H17NO4
- Molecular Weight:263.29
- Hs Code.:2933990090
- European Community (EC) Number:624-510-3
- Mol file:143262-20-8.mol
Synonyms:143262-20-8;N-Boc-indoline-7-carboxylic acid;1-(tert-Butoxycarbonyl)indoline-7-carboxylic acid;1-[(2-methylpropan-2-yl)oxycarbonyl]-2,3-dihydroindole-7-carboxylic acid;N-Boc-Indoline-7-CarboxylicAcid;SCHEMBL2109995;SUAMIYWLXFROHE-UHFFFAOYSA-N;MFCD04973983;AKOS015918191;SB64455;N-Boc-indoline-7-carboxylic acid, 90%;AC-27807;AS-32988;1-(tert-Butoxycarbonyl)indoline-7-carboxylicacid;EN300-1603765;1-(tert-butoxycarbonyl)-7-indolinecarboxylic acid;Q-102604;1-[(tert-butoxy)carbonyl]-2,3-dihydro-1H-indole-7-carboxylic acid;(S)-(+)-3,3'-Bis(3,5-bis(trifluoromethyl)phenyl)-1,1'-binaphthyl-2,2'-diyl hydrogenphosphate, 95%


 Xi,
Xi, N
N


