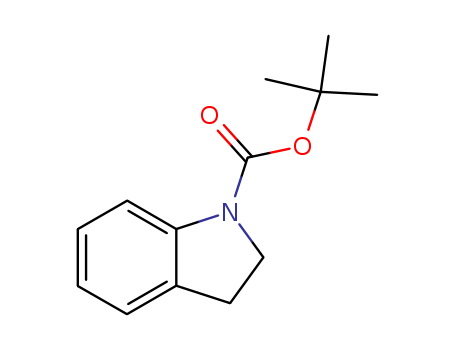
- Chemical Name:tert-Butyl indoline-1-carboxylate
- CAS No.:143262-10-6
- Molecular Formula:C13H17 N O2
- Molecular Weight:219.283
- Hs Code.:2933990090
- European Community (EC) Number:623-971-8
- DSSTox Substance ID:DTXSID30394808
- Nikkaji Number:J914.702K
- Wikidata:Q82194435
- Mol file:143262-10-6.mol
Synonyms:tert-Butyl indoline-1-carboxylate;143262-10-6;1-BOC-INDOLINE;Tert-butyl 2,3-dihydroindole-1-carboxylate;1H-Indole-1-carboxylic acid, 2,3-dihydro-, 1,1-dimethylethyl ester;2,3-dihydroindole-1-carboxylic acid tert-butyl ester;tert-butyl 1-indolinecarboxylate;1-t-butyloxycarbonylindoline;N-(t-butoxycarbonyl)indoline;SCHEMBL552069;1-(tert-butoxycarbonyl)indoline;tert-Butylindoline-1-carboxylate;GWAXLDLPPZPQLO-UHFFFAOYSA-;DTXSID30394808;GWAXLDLPPZPQLO-UHFFFAOYSA-N;MFCD01318399;AKOS009158345;SB64104;tert-Butyl indoline-1-carboxylate, 98%;tert-Butyl?3-oxopyrrolidine-1-carboxylate;CS-0082136;FT-0607418;EN300-127043;F17563;tert-butyl 2,3-dihydro-1H-indole-1-carboxylate;A808047;A838402;J-007784;1,1-dimethylethyl 2,3-dihydro-1H-indole-1-carboxylate;2,3-dihydro-1H-indole-1-carboxylic acid tert-butyl ester;InChI=1/C13H17NO2/c1-13(2,3)16-12(15)14-9-8-10-6-4-5-7-11(10)14/h4-7H,8-9H2,1-3H3