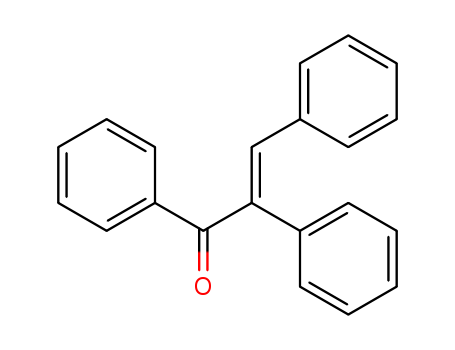
- Chemical Name:(Z)-alpha,beta-Diphenylacrylophenone
- CAS No.:7474-65-9
- Molecular Formula:C21H16O
- Molecular Weight:284.357
- Hs Code.:
- NSC Number:401881,401879,401204
- Nikkaji Number:J1.516.901J
- ChEMBL ID:CHEMBL147559
- Mol file:7474-65-9.mol
Synonyms:MLS003171498;NSC401204;CHEMBL147559;(Z)1,2,3-Triphenyl-propenone;(Z)-alpha,beta-Diphenylacrylophenone;NSC401879;NSC401881;NSC-401204;NSC-401879;NSC-401881;4023-77-2;7474-65-9;7512-67-6