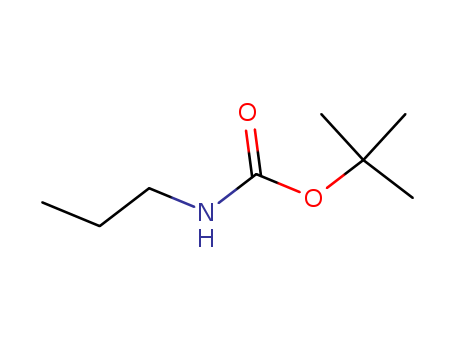
- Chemical Name:tert-Butyl propylcarbamate
- CAS No.:105678-25-9
- Molecular Formula:C8H17 N O2
- Molecular Weight:159.228
- Hs Code.:
- DSSTox Substance ID:DTXSID70557576
- Nikkaji Number:J406.005I
- Wikidata:Q82439525
- Mol file:105678-25-9.mol
Synonyms:tert-Butyl propylcarbamate;105678-25-9;tert-butyl N-propylcarbamate;t-Butyl propylcarbamate;tert-Butylpropylcarbamate;SCHEMBL61208;Carbamic acid, propyl-, 1,1-dimethylethyl ester (9CI);DTXSID70557576;MFCD20763435;AKOS012456138;CS-0335500