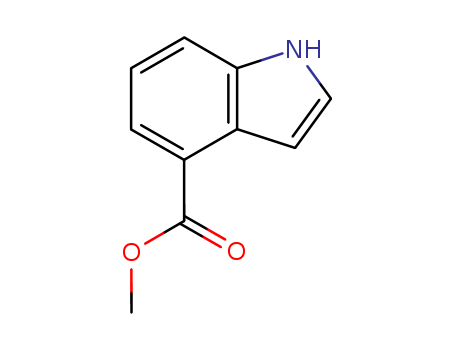
- Chemical Name:methyl 1H-indole-4-carboxylate
- CAS No.:39830-66-5
- Molecular Formula:C10H9NO2
- Molecular Weight:175.187
- Hs Code.:29339900
- European Community (EC) Number:622-802-5
- DSSTox Substance ID:DTXSID80960395
- Nikkaji Number:J258.257K
- Wikidata:Q27454827
- ChEMBL ID:CHEMBL3416133
- Mol file:39830-66-5.mol
Synonyms:Methyl indole-4-carboxylate;39830-66-5;methyl 1H-indole-4-carboxylate;Indole-4-carboxylic Acid Methyl Ester;MFCD00191222;1H-indole-4-carboxylic acid methyl ester;Methyl 4-indolecarboxylate;1H-Indole-4-carboxylic acid, methyl ester;4-METHOXYCARBONYLINDOLE;4-indolecarboxylic acid methyl ester;101277-72-9;4ME;Indole-4-carboxylate;methyl indole4-carboxylate;methylindole-4-carboxylate;methyl indol-4-carboxylate;methyl 4-indole carboxylate;4-methoxycarbonyl-1h-indole;SCHEMBL45030;methyl 1H-4-indolecarboxylate;Methyl 1H-indol-4-carboxylate;methyl 1H-indole 4-carboxylate;CHEMBL3416133;BDBM93017;WEAXQUBYRSEBJD-UHFFFAOYSA-;DTXSID80960395;4b35;WEAXQUBYRSEBJD-UHFFFAOYSA-N;Methyl indole-4-carboxylate, 99%;BCP26865;CS-D1452;indole-4-carboxylic-acid-methylester;BBL013278;STK503877;AKOS000266632;AC-2619;PB32168;SS-6025;SY002750;AM20040378;FT-0618756;FT-0654755;M1773;EN300-217411;I-2510;Q-102106;Q27454827;InChI=1/C10H9NO2/c1-13-10(12)8-3-2-4-9-7(8)5-6-11-9/h2-6,11H,1H3;4-Indole-carboxylic acid methyl ester;Indole-4-carboxylic acid methyl ester


 Xi
Xi


