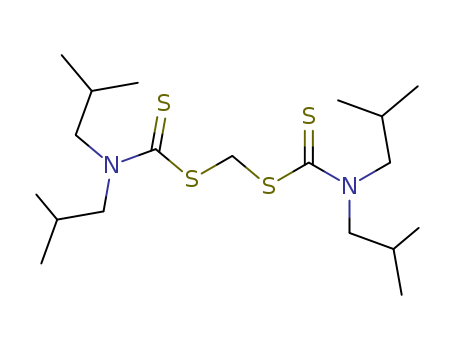
- Chemical Name:Lead ionophore II
- CAS No.:90276-58-7
- Molecular Formula:C19H38N2S4
- Molecular Weight:422.788
- Hs Code.:
- DSSTox Substance ID:DTXSID10400456
- Nikkaji Number:J588.609K
- Wikidata:Q82203448
- Mol file:90276-58-7.mol
Synonyms:Lead ionophore II;90276-58-7;bis(2-methylpropyl)carbamothioylsulfanylmethyl N,N-bis(2-methylpropyl)carbamodithioate;SCHEMBL6932250;DTXSID10400456;Lead ionophore II, Selectophore(TM);methylene bis(diisobutylcarbamodithioate);Bis(diisobutyldithiocarbamic acid)methylene ester;S,S-METHYLENEBIS(N,N-DIISOBUTYLDITHIOCARBAMATE)