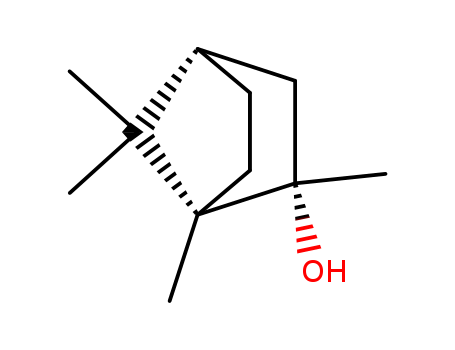
- Chemical Name:(1S)-Exo-1,2,7,7-tetramethylbicyclo[2.2.1]heptan-2-OL
- CAS No.:68330-44-9
- Molecular Formula:C11H20O
- Molecular Weight:168.279
- Hs Code.:
- Nikkaji Number:J664.546A
- Wikidata:Q105151228
- Mol file:68330-44-9.mol
Synonyms:(1S)-EXO-1,2,7,7-TETRAMETHYLBICYCLO[2.2.1]HEPTAN-2-OL;(1S,2S,4S)-2-Methylbornane-2-ol;AKOS006287223