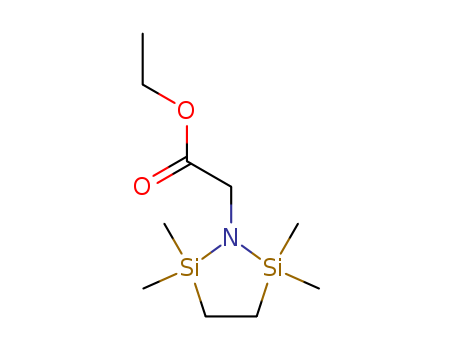
- Chemical Name:Ethyl 2-(2,2,5,5-tetramethyl-1,2,5-azadisilolidin-1-yl)acetate
- CAS No.:78605-23-9
- Molecular Formula:C10H23 N O2 Si2
- Molecular Weight:245.469
- Hs Code.:
- European Community (EC) Number:625-072-6
- Nikkaji Number:J364.554A
- Mol file:78605-23-9.mol
Synonyms:78605-23-9;Ethyl 2-(2,2,5,5-tetramethyl-1,2,5-azadisilolidin-1-yl)acetate;SCHEMBL7903187;NTAZFMGCNVXERO-UHFFFAOYSA-N;2,2,5,5-Tetramethyl-1,2,5-azadisiloiidin-1-acetic acid ethyl ester;FT-0711239;Ethyl 2,2,5,5-tetramethyl-1,2,5-azadisilolidin-1-acetate, >=98.0%;2,2,5,5-tetramethyl-1-aza-2,5-disilacyclopentane-1-acetic acid ethyl ester