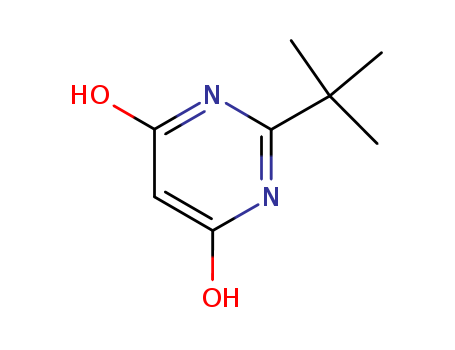
- Chemical Name:2-Tert-butyl-4,6-dihydroxypyrimidine
- CAS No.:18378-79-5
- Molecular Formula:C8H12N2O2
- Molecular Weight:168.195
- Hs Code.:2933599090
- DSSTox Substance ID:DTXSID40355788
- Wikidata:Q82134829
- Mol file:18378-79-5.mol
Synonyms:18378-79-5;2-TERT-BUTYL-4,6-DIHYDROXYPYRIMIDINE;2-tert-butyl-4-hydroxy-1H-pyrimidin-6-one;2-(tert-butyl)pyrimidine-4,6-diol;SCHEMBL1221655;4(3H)-Pyrimidinone,2-(1,1-dimethylethyl)-6-hydroxy-;SCHEMBL21324846;DTXSID40355788;2-(tert-Butyl)-4,6-pyrimidinediol;AKOS012922321;AKOS015909237;CS-0432823;FT-0617253;2-(tert-Butyl)-6-hydroxypyrimidin-4(3H)-one