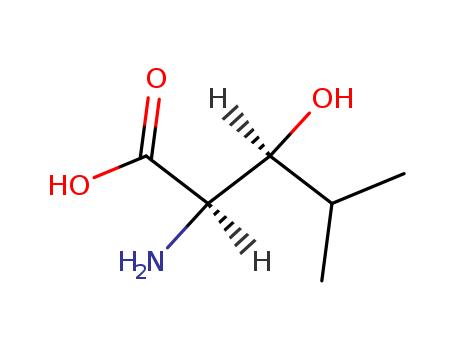
- Chemical Name:(2R,3S)-2-amino-3-hydroxy-4-methylpentanoic acid
- CAS No.:87421-23-6
- Molecular Formula:C6H13 N O3
- Molecular Weight:147.174
- Hs Code.:2922509090
- DSSTox Substance ID:DTXSID00369265
- Nikkaji Number:J603.007F
- Wikidata:Q72437878
- Mol file:87421-23-6.mol
Synonyms:87421-23-6;(2R,3S)-2-amino-3-hydroxy-4-methylpentanoic acid;D-Leucine, 3-hydroxy-, (3S)-;D(-)-threo-3-Hydroxyleucine;(2R,3S)-(-)-2-Amino-3-hydroxy-4-methylpentanoic acid;35016-62-7;(2R,3S)-rel-2-Amino-3-hydroxy-4-methylpentanoic acid;D-threo-hydroxyleucine;(3S)-3-Hydroxy-D-leucine;(2R,3S)-|A-Hydroxyleucine;MFCD00142970;(d)3-hydroxy-Leucine;starbld0019666;SCHEMBL2030131;DTXSID00369265;ZAYJDMWJYCTABM-UHNVWZDZSA-N;BCP09538;AKOS016842860;AC-4459;AS-67678;A862633;(2R*,3S*)-2-amino-3-hydroxy-4-methyl-pentanoic acid;(2R,3S)-2-Amino-3-hydroxy-4-methylpentanoic acid (H-D-Leu(3S-OH)-OH)