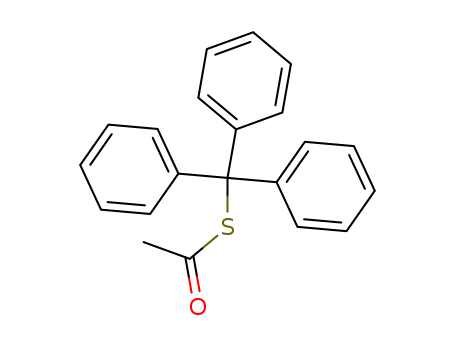
- Chemical Name:S-trityl ethanethioate
- CAS No.:1727-15-7
- Molecular Formula:C21H18 O S
- Molecular Weight:318.439
- Hs Code.:2930909090
- NSC Number:66470
- DSSTox Substance ID:DTXSID00938192
- Nikkaji Number:J2.261.239E
- Wikidata:Q82914454
- ChEMBL ID:CHEMBL261171
- Mol file:1727-15-7.mol
Synonyms:S-trityl ethanethioate;Triphenylmethanethiol acetate;1727-15-7;NSC66470;Thioacetic acid S-trityl ester;CHEMBL261171;BDBM23794;DTXSID00938192;S-(Triphenylmethyl) ethanethioate;Triphenylmethanethiol acetate, 97%;NSC-66470;Triphenylmethyl-containing compound, 23;1-[(triphenylmethyl)sulfanyl]ethan-1-one;J-010844