- Scandium
- Scandium acetate
- Scandium bromide
- Scandium Chloride
- Scandium chloride
- Scandium fluoride
- Scandium hydroxide
- Scandium oxalate (2:3)
- Scandium oxide
- Scandium sulfide
- 12060-58-1Samarium oxide (Sm2O3)
- 12060-59-2Strontium titaniumoxide (SrTiO3)
- 120607-07-0Vicomp alloy
- 12061-16-4Erbium oxide (Er2O3)
- 1206-13-9Cyclohexanecarbonitrile,1-(4-methylphenyl)-
- 120614-25-79H-Imidazo[1,2-a]indol-9-one
- 120-61-61,4-Benzenedicarboxylicacid, 1,4-dimethyl ester
- 12062-24-7Silicate(2-),hexafluoro-, copper(2+) (1:1)
- 1206228-66-14-Piperidinecarboxylic acid, 4-ethyl-, methyl ester
- 1206249-86-6Methyl 4-bromo-6-chloropicolinate
Hot Products
- 1206161-97-8GPLG0634
- 12062-24-7CUPRIC FLUOROSILICATE
- 12063-54-6Iron lanthanum oxide(Fe5La3O12)
- 12068-03-0SODIUM P-TOLUENESULFONATE
- 120712-84-7Formaldehyde, polymer with phenol, potassium salt
- 12072-90-1Hydromagnesite
- 1207314-86-02-bromo-4-(1,1,1,2,3,3,3-heptafluoropropan-2-yl)-6-(trifluoromethyl)aniline
- 120750-67-6BISPHENOL F ETHOXYLATE (2 EO/PHENOL) DIACRYLATE
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin

|
Basic Information |

|
Post buying leads |

|
Suppliers |

| Name |
Scandium oxide |
EINECS | 235-042-0 |
| CAS No. | 12060-08-1 | Density | 8.35 g/mL at 25 °C(lit.) |
| PSA | 43.37000 | LogP | -0.30600 |
| Solubility | insoluble in water | Melting Point |
1000 °C |
| Formula | O3Sc2 | Boiling Point | N/A |
| Molecular Weight | 137.91 | Flash Point | N/A |
| Transport Information | N/A | Appearance | White powder |
| Safety | 24/25 | Risk Codes | N/A |
| Molecular Structure |
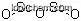
|
Hazard Symbols | N/A |
| Synonyms |
Discandiumtrioxide;Scandia;Scandia (Sc2O3);Scandium oxide (ScO1.5);Scandium sesquioxide;Scandium trioxide;Scandium(3+) oxide;Scandium(III)oxide; |
Scandium oxide Specification
The Scandium oxide, with the cas registry number 12060-08-1, has the IUPAC name of oxygen(2-); scandium(3+). This is a kind of white powder, and is insoluble in water while soluble in hot acid. When you are dealing with this chemical, you should avoid contacting with eyes and skin. What's more, its product categories are including Inorganics; ScandiumMetal and Ceramic Science; Catalysis and Inorganic Chemistry; Chemical Synthesis; Oxides; Scandium.
The physical properties of this chemical are as below: (1)#H bond acceptors: 3; (2)#H bond donors: 0; (3)#Freely Rotating Bonds: 0; (4)Polar Surface Area: 0; (5)Exact Mass: 137.896564; (6)MonoIsotopic Mass: 137.896564; (7)Topological Polar Surface Area: 3; (8)Heavy Atom Count: 5; (9)Covalently-Bonded Unit Count: 5.
The production method of this chemical is below: First get the crude scandium oxide from seperating the Nickel Ore, and then have refining to get scandium hydroxide; Next heat this chemical and then burn to get this products.
As to its usage, it is widely applied in many fields. It could be used as evaporation material of semiconductor cladding material and in preparation of solid-state laser and metal halide lamp; It could be used in electronic industry, laser light & superconducting material, alloy addition, cathode coating additives and so on.
In addition, you could obtain the molecular structure by using the following datas:
(1)Canonical SMILES: [O-2].[O-2].[O-2].[Sc+3].[Sc+3]
(2)InChI: InChI=1S/3O.2Sc/q3*-2;2*+3
(3)InChIKey: HJGMWXTVGKLUAQ-UHFFFAOYSA-N

