Products Categories
| CAS No.: | 100-01-6 |
|---|---|
| Name: | 4-Nitroaniline |
| Article Data: | 828 |
| Molecular Structure: | |
|
|
|
| Formula: | C6H6N2O2 |
| Molecular Weight: | 138.126 |
| Synonyms: | Aniline,p-nitro- (8CI);1-Amino-4-nitrobenzene;4-Amino-1-nitrobenzene;4-Aminonitrobenzene;4-Nitro-1-aminobenzene; |
| EINECS: | 202-810-1 |
| Density: | 1.333 g/cm3 |
| Melting Point: | 147 °C |
| Boiling Point: | 333.1 °C at 760 mmHg |
| Flash Point: | 165 °C |
| Solubility: | 0.8 g/L (20 °C) in water |
| Appearance: | Yellow solid with a mild odor |
| Hazard Symbols: |
 T, T, F F
|
| Risk Codes: | 23/24/25-33-52/53-39/23/24/25-11 |
| Safety: | 28-36/37-45-61-28A-16-1/2-7 |
| Transport Information: | UN 1661 |
| PSA: | 71.84000 |
| LogP: | 2.28140 |
- 144851-82-1METHYL2-AMINO-3-FLUOROBENZOATE
- 483366-12-7(2S,4R)-1-Boc-2-cyano-4-hydroxypyrrolidine
- 173606-50-3BOC-10-AMINODECANOIC ACID
- 361456-36-2METHYL (R)-(+)-ISOCYANATO-3-PHENYLPROPI&
- 5156-58-1N-(1-Benzyl-4-pipperidinyl)-N-phenylpropanamide HCl
- 81281-59-67-Benzylideneaminotheophylline
- 50288-62-5threo-Phenyl-2-piperidyl acetamide
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 47087-37-6Z-D-Glu-OMe
- 1219080-61-1IMIDAZOLE-2-BORONIC ACID

| Conditions | Yield |
|---|---|
| With formic acid In acetonitrile at 20℃; for 1h; Irradiation; | 100% |
| In water at 25℃; for 0.333333h; Sonication; | 99% |
| With sodium tetrahydroborate In water at 95℃; for 0.333333h; Green chemistry; | 98% |

| Conditions | Yield |
|---|---|
| With 1,1,1,3',3',3'-hexafluoro-propanol; tetrabutylammonium perchlorate In N,N-dimethyl-formamide electrolytic reduction at a Pt gauze electrode at a potential of -0.30 V; | 100% |
| With Decaborane; palladium on activated charcoal In methanol at 20℃; for 0.25h; | 99% |
| With triethylsilane; indium(III) chloride In acetonitrile at -20℃; for 0.25h; | 99% |
- 77853-01-1
(4-nitro-phenyl)-m-tolyl sulfone

A
- 100-32-3
di(p-nitrophenyl) disulfide

B
- 77853-09-9
Toluene-3-thiosulfonic acid; compound with ammonia

C
- 100-01-6
4-nitro-aniline

| Conditions | Yield |
|---|---|
| With sulfur; ammonia at 100℃; for 2h; titanium autoclave; | A 43% B 100% C 40% |

- 22865-57-2
1-methoxy-4-((4-nitrophenyl)sulfonyl)benzene

A
- 100-32-3
di(p-nitrophenyl) disulfide

B
- 77853-03-3
4-Methoxy-benzenethiosulfonic acid; compound with ammonia

C
- 100-01-6
4-nitro-aniline

| Conditions | Yield |
|---|---|
| With sulfur; ammonia at 100℃; for 2h; titanium autoclave; | A 56% B 100% C 32% |
| With sulfur; ammonia at 100℃; for 2h; Product distribution; titanium autoclave; various reactions temp. and amounts of S8; |


| Conditions | Yield |
|---|---|
| With graphitic carbon nitride; hydrazine hydrate In water at 70℃; for 8h; Time; Darkness; Sealed tube; Green chemistry; chemoselective reaction; | 100% |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide at 120 - 190℃; under 45004.5 - 46504.7 Torr; for 4h; Temperature; Pressure; | 99.31% |
| With ammonium hydroxide; copper(l) iodide at 200℃; for 6.5h; Reagent/catalyst; Temperature; Time; Autoclave; Green chemistry; | 97% |
| With copper(ll) sulfate pentahydrate; ammonium hydroxide In PEG1000-DIL; methyl cyclohexane at 60℃; for 4h; | 96% |
- 18437-63-3
tert-butyl N-(4-nitrophenyl)carbamate

- 100-01-6
4-nitro-aniline

| Conditions | Yield |
|---|---|
| With EPZG clay In dichloromethane for 1.5h; deacylation; Heating; | 99% |
| With H-β zeolite In dichloromethane for 4h; Heating; | 98% |
| With water at 150℃; for 10h; Subcritical conditions; | 97% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; ascorbic acid In ammonia at 25℃; for 18h; Kinetics; liquid NH3; | 99% |
| With ammonium hydroxide; copper(l) iodide; phosphate potassium salt In N,N-dimethyl-formamide at 20℃; for 36h; Inert atmosphere; | 98% |
| Stage #1: p-nitrobenzene iodide With copper(l) iodide; D-glucosamine hydrochloride; potassium carbonate In water; acetone at 90℃; for 0.166667h; Stage #2: With ammonia In water; acetone at 90℃; for 28h; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: para-nitrophenyl bromide With copper(l) iodide; D-glucosamine hydrochloride; potassium carbonate In water; acetone at 90℃; for 0.166667h; Stage #2: With ammonia In water; acetone at 90℃; for 28h; | 98% |
| With ammonium hydroxide at 20℃; for 3h; Catalytic behavior; | 98% |
| With ammonia; triethylamine In water at 20℃; for 2.5h; | 98% |
- 63-68-3
L-methionine

- 74197-40-3
3,5-dinitro-1-(4-nitrophenyl)-4-pyridone

A
- 100-01-6
4-nitro-aniline

B
- 92782-41-7
(S)-2-(3,5-Dinitro-4-oxo-4H-pyridin-1-yl)-4-methylsulfanyl-butyric acid

| Conditions | Yield |
|---|---|
| In pyridine; water for 3h; Ambient temperature; | A n/a B 98% |

 This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
Specification
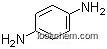
The 4-Nitroaniline with CAS registry number of 100-01-6 is also known as 1-Amino-4-nitrobenzene. The IUPAC name and product name are the same. It belongs to product categories of Anilines, Aromatic Amines and Nitro Compounds; Organics; Aniline; Functional Materials; Organic Nonlinear Optical Materials; NA - NI; Alpha Sort; Amines; AromaticsVolatiles/ Semivolatiles; Chemical Class; N; N-OAlphabetic; Analytical Standards; AromaticsChemical Class; NA - NIAnalytical Standards; Nitro Compounds; Indicators for non-aqueous titrationsTitration;Metal Titration Indicators; Indicators; Titration; I-N, Puriss p.a.Spectroscopy; Analytical Reagents for General Use; Mass Spectrometry (MS)&LC-MS; Puriss p.a.; Reagents for Mass Spectrometry (MS); C2 to C6Photonic and Optical Materials; NLO Chromophores and Intermediates; Non-Linear Optical (NLO) Materials; C2 to C6Stains and Dyes; Nitrogen Compounds; Stains&Dyes, A to. Its EINECS registry number is 202-810-1. In addition, the formula is C6H6N2O2 and the molecular weight is 138.12. This chemical is a yellow solid with a mild odor and should be sealed in ventilated, cool room away from fire and heat. Besides, it is slightly soluble in cold water, soluble in boiling water, ethanol, ether, benzene and acid.
Physical properties about 4-Nitroaniline are: (1)ACD/LogP: 1.20; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.195; (4)ACD/LogD (pH 7.4): 1.195; (5)ACD/BCF (pH 5.5): 4.769; (6)ACD/BCF (pH 7.4): 4.769; (7)ACD/KOC (pH 5.5): 106.469; (8)ACD/KOC (pH 7.4): 106.472; (9)#H bond acceptors: 4; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 2; (12)Index of Refraction: 1.634; (13)Molar Refractivity: 37.034 cm3; (14)Molar Volume: 103.555 cm3; (15)Surface Tension: 60.318 dyne/cm; (16)Density: 1.334 g/cm3; (17)Flash Point: 165 °C; (18)Enthalpy of Vaporization: 57.588 kJ/mol; (19)Boiling Point: 333.051 °C at 760 mmHg; (20)Vapour Pressure: 0 mmHg at 25 °C.
Preparation of 4-Nitroaniline: it is prepared by nitration, hydrolysis reaction of acetanilide.
C6H4ClNO2 + 2 NH3 → NH4Cl + C6H6N2O2
Uses of 4-Nitroaniline. This chemical is commonly used as an intermediate in the synthesis of dyes, antioxidants, pharmaceuticals and gasoline, in gum inhibitors, poultry medicines, and as a corrosion inhibitor. What's more, it can be used to produce 1-(4-nitro-phenyl)-pyrrole-2-carbaldehyde by reaction with furfural. The reaction occurs with reagent 2n-HCl and solution ethanol with other condition of heating for 2 hours. The yield is about 90%.

When you are using this chemical, please be cautious about it. As a chemical, it is harmful to aquatic organisms that may cause long-term adverse effects in the aquatic environment. Beside, it has danger of very serious irreversible effects through inhalation, in contact with skin and if swallowed. Furthermore, it has danger of cumulative effects and it is highly flammable. During using it, wear suitable protective clothing, gloves and keep away from sources of ignition. In case of accident or if you feel unwell seek medical advice immediately. After contact with skin, wash immediately. Afer using it, keep container tightly closed, locked up and out of the reach of children. Avoid release to the environment.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: C1=CC(=CC=C1N)[N+](=O)[O-]
2. InChI: InChI=1S/C6H6N2O2/c7-5-1-3-6(4-2-5)8(9)10/h1-4H,7H2
3. InChIKey: TYMLOMAKGOJONV-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| bird - wild | LD50 | oral | 75mg/kg (75mg/kg) | Toxicology and Applied Pharmacology. Vol. 21, Pg. 315, 1972. | |
| guinea pig | LD50 | oral | 450mg/kg (450mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Voprosy Kommunal'noi Gigieny. Problems of Communal Hygiene. Vol. 6, Pg. 89, 1966. |
| guinea pig | LD50 | skin | > 500mg/kg (500mg/kg) | National Technical Information Service. Vol. OTS0572389, | |
| mammal (species unspecified) | LDLo | intravenous | 40mg/kg (40mg/kg) | BLOOD: METHEMOGLOBINEMIA-CARBOXYHEMOGLOBIN | U.S. Public Health Service, Public Health Bulletin. Vol. 271, Pg. 34, 1941. |
| mouse | LD50 | intramuscular | 800mg/kg (800mg/kg) | Igiena. Vol. 15, Pg. 151, 1966. | |
| mouse | LD50 | intraperitoneal | 250mg/kg (250mg/kg) | National Technical Information Service. Vol. AD691-490, | |
| mouse | LD50 | oral | 810mg/kg (810mg/kg) | Toxicology and Applied Pharmacology. Vol. 42, Pg. 417, 1977. | |
| quail | LD50 | oral | 1gm/kg (1000mg/kg) | Archives of Environmental Contamination and Toxicology. Vol. 12, Pg. 355, 1983. | |
| rat | LD50 | oral | 750mg/kg (750mg/kg) | Ceskoslovenska Hygiena. Czechoslovak Hygiene. Vol. 23, Pg. 168, 1978. | |
| rat | LDLo | intraperitoneal | 600mg/kg (600mg/kg) | "Toxicometric Parameters of Industrial Toxic Chemicals Under Single Exposure," Izmerov, N.F., et al., Moscow, Centre of International Projects, GKNT, 1982Vol. -, Pg. 92, 1982. |